Clinical Features

Symptoms of Haemolysis. The presenting symptoms depend on the severity of the anaemia and the ability of the patient to compensate. Patients often experience fatigue, dizziness or dyspnoea on exertion. In severe anaemia,there may be dyspnoea at rest or chest pain. Patients may also report jaundice, pale stools or tea-coloured urine.
Pathophysiology of AIHA

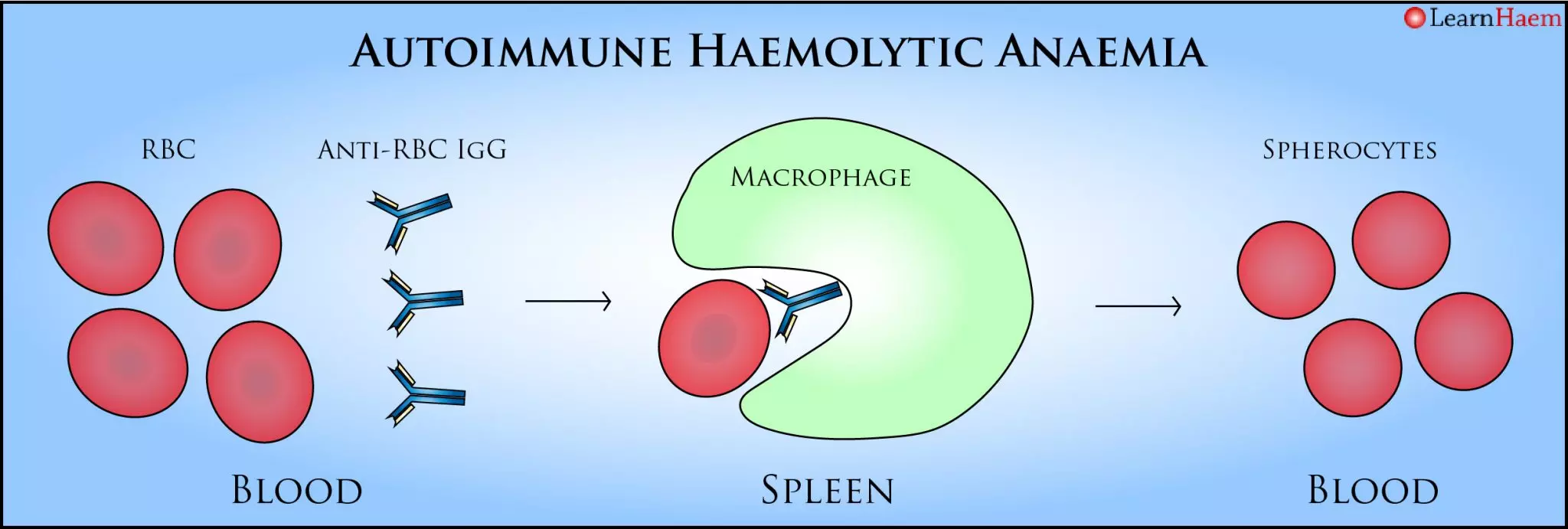
Warm Autoimmune Haemolytic Anaemia. In warm AIHA, the body produces an IgG autoantibody directed against a red cell antigen. This binds at body temperature, and results in opsonisation of the red cell. When the opsonised red cell encouters macrophages in the spleen, they are partly phagocytosed, resulting in membrane damage and haemolysis. The membrane damage is what gives RBCs a spherical appearance on the peripheral blood film.
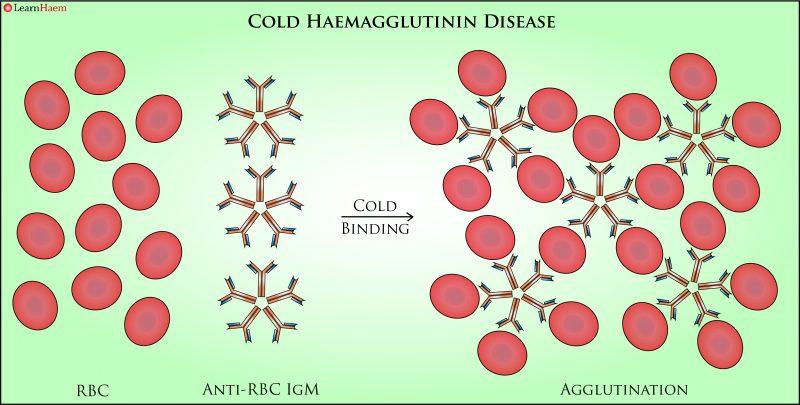
Cold Haemagglutinin Disease. In CHAD, the offending antibody is an IgM antibody which is more active at cold temperatures. Because IgM exists as a pentamer, its binding causes direct agglutination of RBCs.
Causes of Warm AIHA
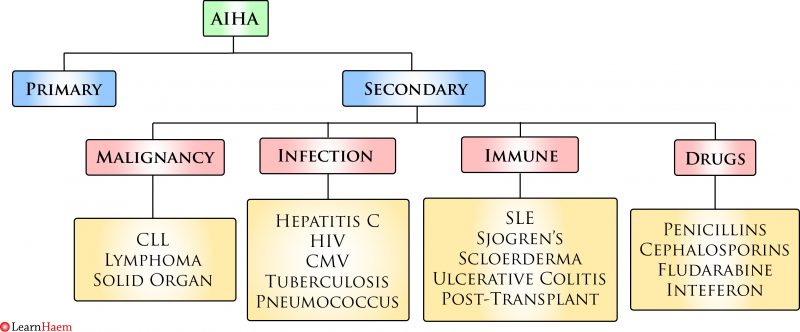
Causes of Warm AIHA. CLL: chronic lymphocytic leukaemia, HIV: human immunodeficiency virus, CMV: cytomegalovirus, SLE: systemic lupus erythematosus.
Management of Warm AIHA
If a patient with AIHA presents with acute, severe haemolysis, the first-line treatment is steroids. Often, prednisolone at a dose of 1mg/kg is given. Transfusion is often necessary for symptomatic anaemia. The blood bank requires time to perform an antibody screen to rule out clinically-significant alloantibodies. However, in AIHA, this will take a lot of time because of the autoantibody coating the red cells. Recall from the section on the Coombs test that Coombs reagent will react whenever there is an antibody adherent to the red cell surface. Hence, patients with AIHA often have a “pan-reactive” screening cell panel when screening for an alloantibody. The autoantibody does not usually interfere with ABO grouping because they are not direct agglutinins (in contrast to ABO antibodies, which are IgM antibodies). In order to identify alloantibodies, the blood bank will first need to carry out an auto-adsorption procedure, shown below. As the auto-adsorption process takes time, in an emergency situation, the patient should be supplied with ABO, Rhesus D-matched blood, without waiting for the antibody screen.

Auto-Adsorption. In order to identify any alloantibodies, a process known as auto-adsorption needs to be carried out. The premise of this test is that alloantibodies will not react with the patient’s own cells (individuals can only make alloantibodies to an antigen that they do not have). This test cannot be done if the patient has been recently transfused, as there will be donor red cells in the circulation, which could adsorb any alloantibodies. 1. The patient’s RBCs are treated to remove any bound autoantibody. 2. They are then incubated with the patient’s plasma, which results in the autoantibody binding to the patient’s red cells. This process is repeated until all the autoantibody from the plasma is adsorbed out, leaving behind only alloantibodies to be identified. 3. A DCT should be done on the patient’s red cells after the final adsorption – it must be negative to ensure all the autoantibody has been adsorbed out of the plasma.

Leave A Comment