α Thalassaemia
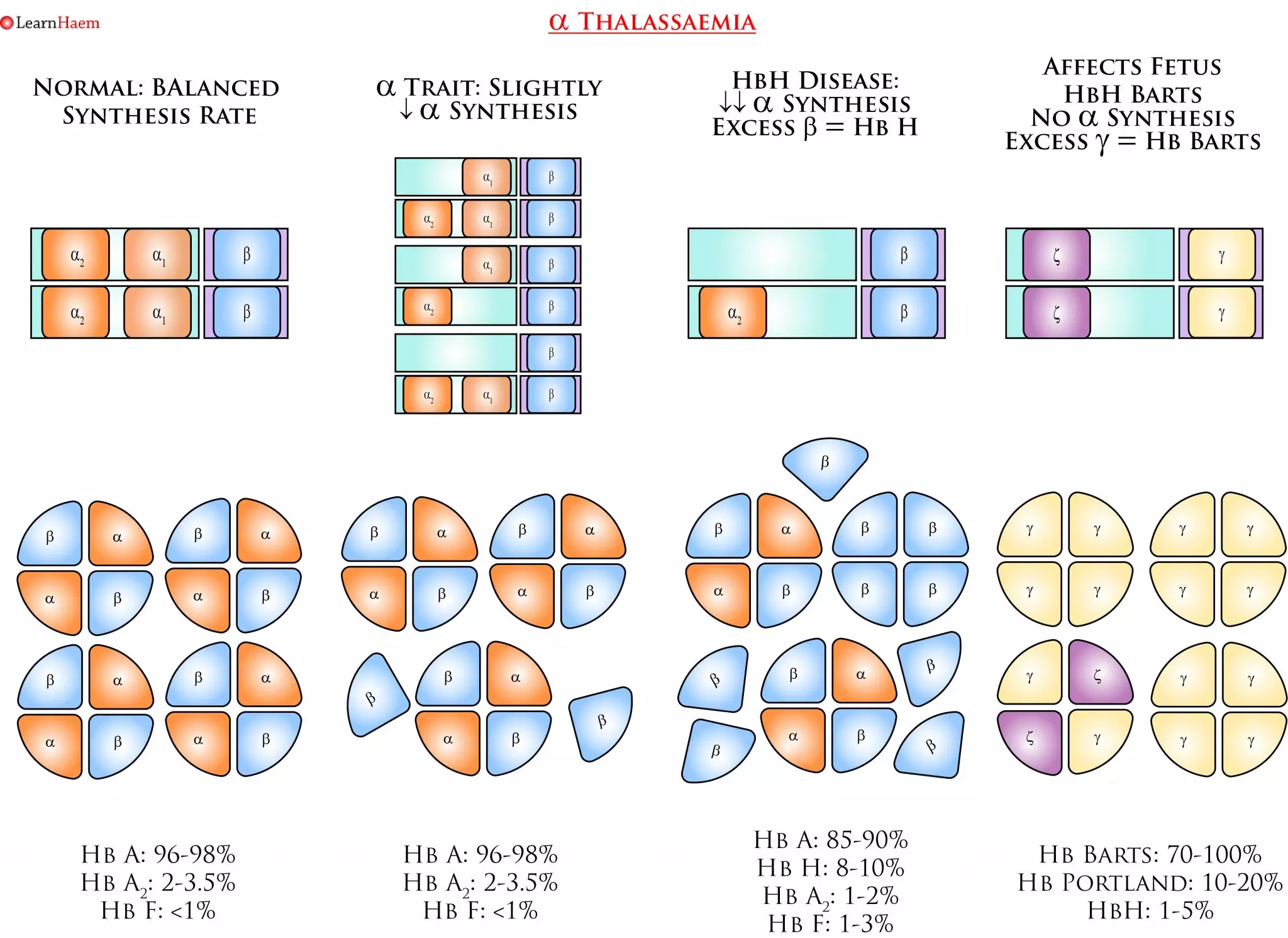
α thalassaemia results from deletions in the α globin gene. A normal human has 4 α globin genes. Deletions in α genes result in imbalanced synthesis of α globins and ß globins. In more severe disease (–/-α or –/–), the excess ß globins for HbH (ß4) and Hb Barts (γ4) respectively. These haemoglobins have an extremely high oxygen affinity and are unstable, precipitating in red blood cells as they age. This results in membrane damage and markedly reduced red cell survival. The high oxygen affinity of HbH and Hb Barts means they are ineffective as oxygen delivery devices.
Pathophysiology
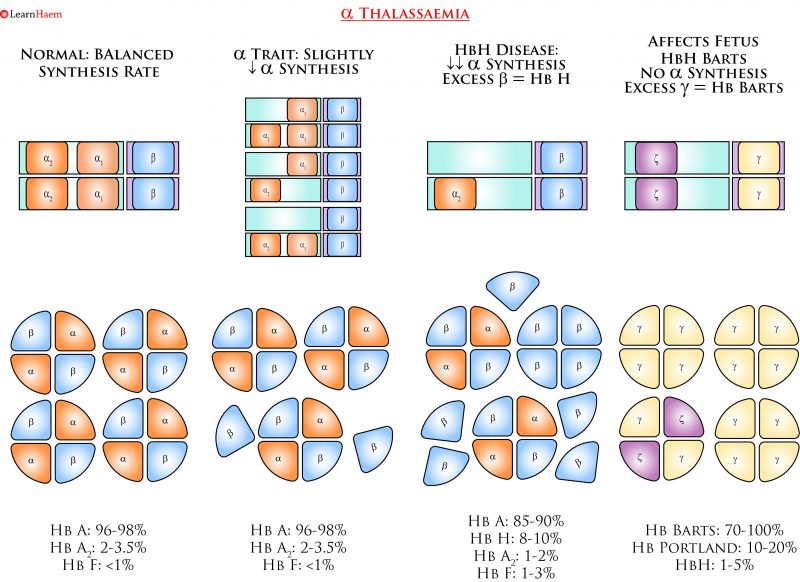
α Thalassaemia. Deletions in the α globin gene result in an imbalance in α and ß chain synthesis. As a result, there is excess formation of ß chains. In more severe phenotypes, these form tetramers of ß4 known as HbH, which is unstable and has an extremely high oxygen affinity. In utero, where γ globin predominates, Hb Barts (γ4) is formed.
Clinical Phenotypes

α Thalassaemia Nomenclature. This is a simplified example of the various genotypes which can lead to the various clinical phenotypes seen in α thalassaemia. α thalassaemia trait occurs when either one or two α genes are deleted. The clinical phenotype is mild, ranging from a normal full blood count, to a mild anaemia with microcytosis. Diagnosis of α thalassaemia using haemoglobin electrophoresis or by looking for HbH bodies is unreliable, and genetic studies should be sent when there are clinical implications (e.g. pre-conception planning). HbH disease results from a three-gene deletion. It is characterised by HbH bodies in RBCs, which are most easily seen when a brilliant cresyl blue stain is used. Hb Barts disease is not compatible with life – fetuses often die in utero or shortly after birth.
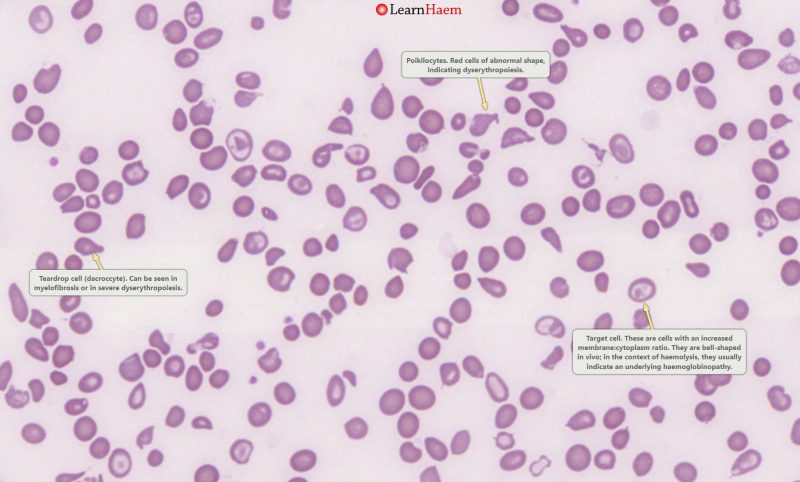
Peripheral Blood Film. This is a blood film from a patient with HbH disease. There are poikilocytes (abnormally-shaped cells), target cells and teardrop cells, which signifies marked dyserythropoiesis.
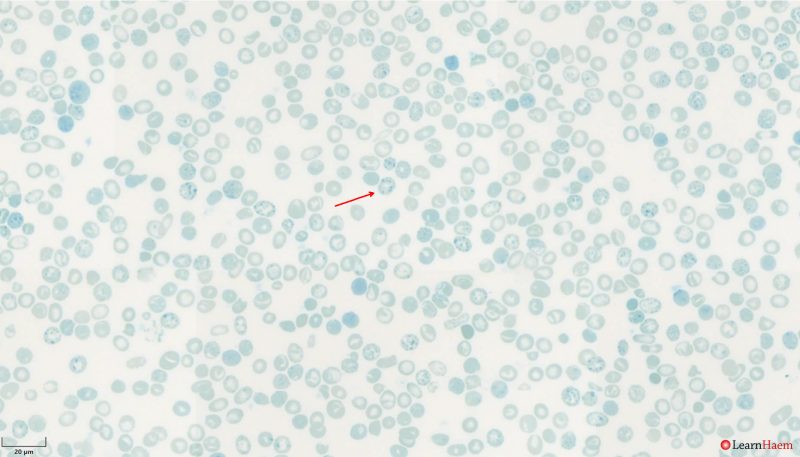
HbH Bodies. These are seen using supravital stains such as brilliant cresyl blue or new methylene blue, which highlight ribosomal precipitates in red blood cells. This is a film from a patient with HbH disease showing numerous HbH bodies (red arrow).
Inheritance
α thalassaemia is inherited in an autosomal recessive fashion. However, because there are four α genes, the inheritance patterns are a little more complex. Genotyping is almost always essential for pre-conception counseling because the exact genotype is crucial for determining the risk of inheriting a more severe phenotype. In the example below, both sets of parents have α thalassaemia trait. However, the set of parents with α0 trait has a 25% chance of having a baby with Hb Barts disease. This is in contrast to the set of parents with α+ trait, who do not need to worry about having children with a more severe disease phenotype. The last example demonstrates why it is important to know if even one of the parents has α0 trait, because it can interact with α+ trait to result in HbH disease.
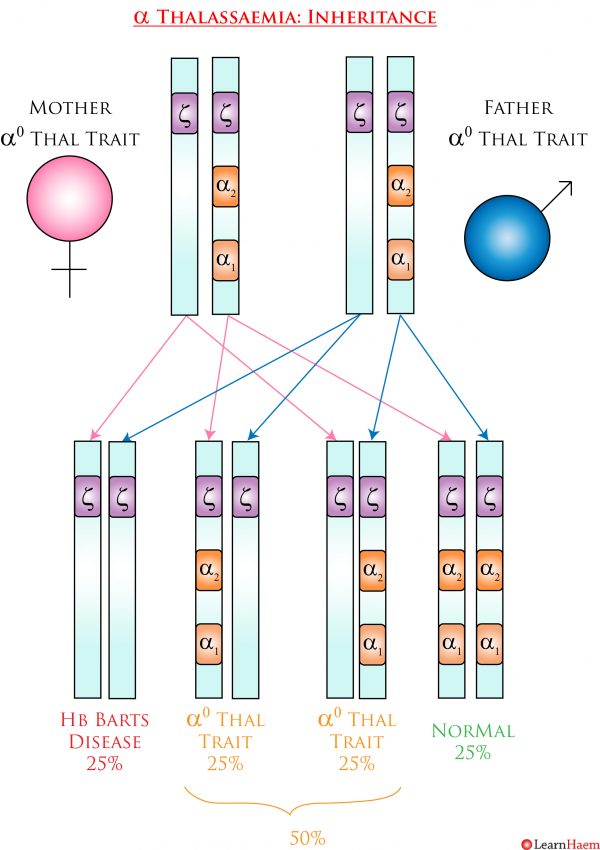
α0 Thalassaemia Trait Inheritance Pattern. In this example, both parents have α thalassaemia trait. However, because they both have two gene deletions which are on the same chromosome, they have a 25% chance of having a child who will have a 4 gene deletion, resulting in Hb Barts disease. 50% of their children will have α0 thalassaemia trait, and 25% will be genotypically normal.

α+ Thalassaemia Trait Inheritance Pattern. In this example, both parents have α thalassaemia trait. However, because have at least one normal α gene on each chromosome, they have have no chance of producing a child with a more severe disease phenotype. All their children will have α+ thalassaemia trait.
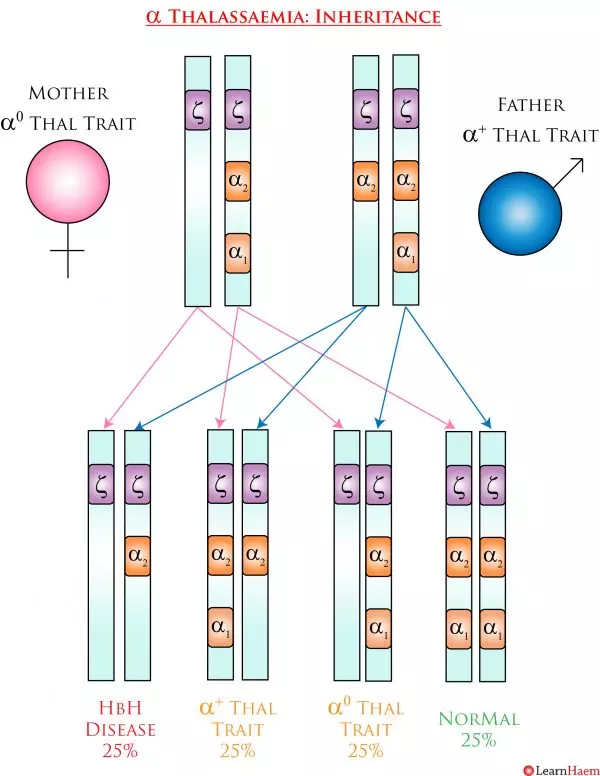
α+ and α0 Trait Inheritance Pattern. In this example, the parents have the same total number of α genes as the couple in the example above. However, because the mother has α0 trait, 25% of the children will have a –/-α genotype, resulting in HbH disease. 50% will have α thalassaemia trait and 25% will be genetically normal.

Leave A Comment