Haemoglobin electrophoresis utilises the charged properties of normal haemoglobin and its variants to replicate characteristic mobility patterns on both alkaline and acidic gels.
Alkaline gel electrophoresis (cellulose acetate membrane, pH 8.4-8.6) is an important method for screening for variant haemoglobins, and is often employed as one of two screening and identification methods (the other being high performance liquid chromatography, HPLC).
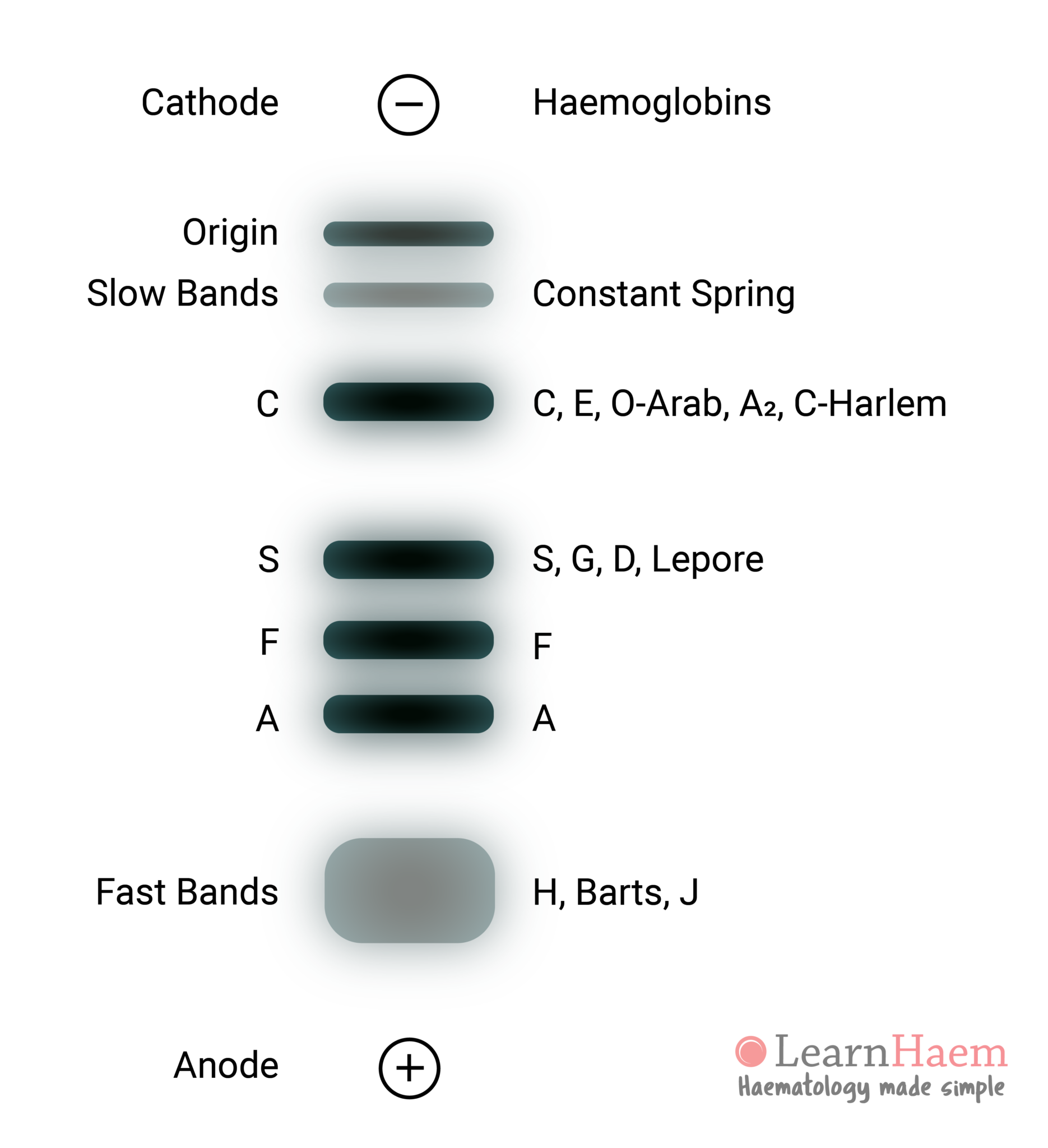
At alkaline pH, haemoglobin is a negatively charged molecule, and hence migrates towards the anode (the positively-charged pole of the gel). Variant haemoglobins have alterations in their surface charge due to changes in surface amino acids. This hence changes the speed of its migration and causes characteristic separation based on set mobility patterns.

Acid agarose gel electrophoresis is available commercially and can complement akaline gel electrophoresis. It is able to separate haemoglobin C from the other haemoglobin variants with the same mobility at alkaline pH (haemoglobins E, O-Arab, A2 and C-Harlem). In addition, it is also able to separate haemoglobin S from haemoglobins G, D and Lepore.


Leave A Comment